Introduction
Current diagnosis of chronic myelomonocytic leukemia (CMML) requires peripheral blood (pb) monocytosis ≥1×109/L. Accordingly, cases which fulfill other diagnostic criteria of CMML but not reaching the required pb monocytosis threshold would be classified as MDS or unclassifiable MPN/MDS according to WHO classification (Arber et al, Blood 2016) Recently, a group of authors (Geyer et al, Modern Pathology 2017) proposed the term oligomonocytic CMML (OM-CMML) for these patients with blood monocytes ≥10% of the WBCs, but only accounting for 0.5-1 × 109/L as an absolute value and fulfilling all other criteria of CMML and suggested that they should be managed as other patients with classical CMML despite lacking pb monocytosis ≥1×109/L.
To address clinical value of this proposed newly entity, we analyzed the incidence, clinico-biological characteristics and outcome of a series of patients fulfilling the proposed criteria for OM-CMML from a single center with a long follow-up.
Methods
We included patients diagnosed between 1997 and 2019 who gathered the proposed criteria for OM-CMML (Geyer et al, Modern Pathology 2017). These patients were compared with a cohort of patients from the same study period diagnosed with classical CMML. Statistical analyses were performed using Rv3.1 and SPSS v20. Next generation targeted sequencing (NGS) was performed with Ion Ampliseq AML Research and Oncomine Myeloid Research Assay panel
Results
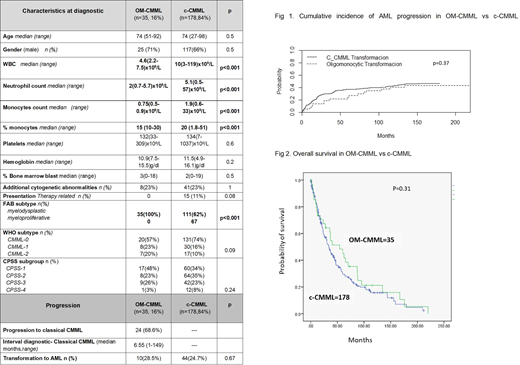
Overall, we included in the study 213 patients, including 35 (16%) who fulfilled the proposed criteria for OM-CMML. Median follow-up of alive patients was 42 months. In the OM-CMML group, 71% were males, median age was 74 years (51-92). OM-CMML patients presented at diagnosis with a lower leucocyte count (WBC) (median value, 4.6(2.2-7.5)x109/L vs 10(3-119)x109/L, p<0.001), neutrophil count (2(0.7-5.7)x109/Lvs5.1(0.5-57)x109/L; p<0.001), and monocyte count, both in terms of absolute figures (0.75(0.5-0.9)x109/Lvs1.9(0.6-33)x109/L;p<0.001) and relative percentage (15% (10-30) vs 20% (1.8-51);p<0.001). All OM-CMML patients corresponded to FAB non-leucocytosis, CMML-Myelodysplastic type (CMML-MD), whereas 62% of c-CMML patients were diagnosed as a CMML-MD subtype p<0.001). No other different clinical characteristic were observed (Table 1). Cytogenetic analysis showed an abnormal karyotype in 23% of OM-CMML patients.
NGS at diagnosis was available in 26 pt, without observing significant differences regarding gene mutation frequency. At diagnosis 17% of OM-CMML patients were transfusion-dependent and the distribution according to CPSS categories was: low (48%), int-1 (23%), int-2 (26%) and high (3%) risk, without difference with c-CMML (Table 1). Progression to a c-CMML was observed in 67% (24) of OM-CMML pts with a median time to progression of 7 months (m) (1-149 m). We did not observe differences in transformation rate to AML (AML-t; 10 (28.5%) vs 44 (24.7%) among OM-CMML and c-CMML group, p=0.6) or cumulative incidence (CI) of AML-t between OM-CMML and c-CMML patients (50m-CI AML-t: 35%±7 vs 21±12, p=0.3) (Fig 1).
Eight out of the 10 pt (80%) who developed an AML previously presented a c-CMML phase. Median time to AML-t was longer in OM-CMML pt: 60m (3-219) vs 13.7m (0.8-124), p=0.011. The percentage of patients who received treatment in OM-CMML cohort was similar to that of c-CMML pts: (28% vs 21%, p=0.37, respectively). Moreover, time to treatment requirement was similar in both patient cohorts (15m (0.9-211m) vs 10m (0.1-112), p=0.5, respectively). Finally, overall survival of OM-CMML did not differ from that of c-CMML (5-year Overall Survival: 45±16% vs 30±7%; p=0.31, Figure 2).
Conclusion:
Clinical features and evolution of patients with OM-CMML were comparable to that of patients with c-CMML, supporting similar classification and management criteria.
Acknowledgement: PI16/01027 (JE; MDB), PI19/01476 (JE; MDB)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal